McqMate
249
100.7k
180+ Pharmaceutical Organic Chemistry 3 Solved MCQs
These multiple-choice questions (MCQs) are designed to enhance your knowledge and understanding in the following areas: Bachelor of Pharmacy (B. Pharma) , Pharmacy .
| 1. |
1 Hexane and 3-methylpentane are examples of: |
| A. | Enantiomers. |
| B. | Stereoisomer. |
| C. | Diastereomers. |
| D. | Constitutional isomers. |
| Answer» D. Constitutional isomers. | |
| 2. |
Cis-trans isomers are: |
| A. | Diastereomers. |
| B. | Enantiomers. |
| C. | Geometric isomers. |
| D. | Constitutional isomers. |
| Answer» C. Geometric isomers. | |
| 3. |
(2R,4S) –2,4–Dichloropentane and (2S,4R)-2,4-dichloropentane are: |
| A. | Enantiomers |
| B. | Diastereomers |
| C. | identical |
| D. | conformational isomers |
| Answer» C. identical | |
| 4. |
Which of the following molecules is achiral? |
| A. | (2R,3R)-2,3-Dichloropentane |
| B. | (2R,3S)-2,3-Dichloropentane |
| C. | (2S,4S)-2,4-Dichloropentane |
| D. | (2S,4R)-2,4-Dichloropentane |
| Answer» D. (2S,4R)-2,4-Dichloropentane | |
| 5. |
Which statement is not true for a meso compound? |
| A. | The specific rotation is 0º. |
| B. | There are one or more planes of symmetry. |
| C. | More than one stereogenic center must be present. |
| D. | The stereo chemical labels, (R) and (S), must be identical for each stereogenic centre. |
| Answer» D. The stereo chemical labels, (R) and (S), must be identical for each stereogenic centre. | |
| 6. |
Which is a meso compound? |
| A. | (2R,3R)-2,3-Dibromobutane |
| B. | (2R,3S)-2,3-Dibromopentane |
| C. | (2R,4R)-2,4-Dibromopentane |
| D. | (2R,4S)-2,4-Dibromopentane |
| Answer» D. (2R,4S)-2,4-Dibromopentane | |
| 7. |
What is the percent composition of a mixture of (S)-(+)-2-butanol,[!] D/25 = +13.52º, and (R)-(-)-2-butanol,[!] D/25 = -13.52º, with a specific rotation [!] D/25 = +6.76º? |
| A. | 75%(R) 25%(S) |
| B. | 25%(R) 75%(S) |
| C. | 50%(R) 50%(S) |
| D. | 67%(R) 33%(S) |
| Answer» B. 25%(R) 75%(S) | |
| 8. |
Which one of the following can exist in optically active forms? |
| A. | cis-1,3-Dichlorocyclohexane |
| B. | trans-1,3-Dichlorocyclohexane |
| C. | cis-1,4-Dichlorocyclohexane |
| D. | tr |
| Answer» D. tr | |
| 9. |
Which of these is a comparatively insignificant factor affecting the magnitude of Specific optical rotation? |
| A. | Concentration of the substance of interest |
| B. | Purity of the sample |
| C. | Temperature of the measurement |
| D. | Length of the sample tube |
| Answer» C. Temperature of the measurement | |
| 10. |
What can be said with certainty if a compound has [!] D/25 = -9.25º ? |
| A. | The compound has the (S) configuration. |
| B. | The compound has the (R) configuration. |
| C. | The compound is not a meso form. |
| D. | The compound possesses only one stereogenic center. |
| Answer» C. The compound is not a meso form. | |
| 11. |
An alkane which can exhibit optical activity is: |
| A. | Neopentane |
| B. | Isopentane |
| C. | 3–Methylpentane |
| D. | 3–Methylhexane |
| Answer» D. 3–Methylhexane | |
| 12. |
In the absence of specific data, it can only be said that (R)–2–bromopentane is: |
| A. | Dextrorotatory (+). |
| B. | Levorotatory (–). |
| C. | Optically inactive. |
| D. | Chiral. |
| Answer» D. Chiral. | |
| 13. |
If a solution of a compound (30.0 g/100 mL of solution) has a measured rotation of +15º in a 2 dm tube, the specific rotation is: |
| A. | +50º |
| B. | +25º |
| C. | +15º |
| D. | +7.5º |
| Answer» B. +25º | |
| 14. |
Which compound would show optical activity? |
| A. | cis 1,4- Dimethylcyclohexane |
| B. | trans 1,4- Dimethylcyclohexane |
| C. | cis 1,4- Dimethylcycloheptane |
| D. | tr |
| Answer» D. tr | |
| 15. |
Of the compounds which correspond to the general name "dichlorocyclobutane", how many are optically active? |
| A. | 0 |
| B. | 1 |
| C. | 2 |
| D. | 3 |
| Answer» C. 2 | |
| 16. |
Which of the following is true of any (S)-enantiomer? |
| A. | It rotates plane-polarized light to the right. |
| B. | It rotates plane-polarized light to the left. |
| C. | It is a racemic form. |
| D. | It is the mirror image of the corresponding (R)-enantiomer. |
| Answer» D. It is the mirror image of the corresponding (R)-enantiomer. | |
| 17. |
Enantiomers are: |
| A. | Molecules that have a mirror image. |
| B. | Molecules that have at least one stereogenic center. |
| C. | Non-superposable molecules. |
| D. | Non-superposable molecules that are mirror images of each other. |
| Answer» D. Non-superposable molecules that are mirror images of each other. | |
| 18. |
Which of the following is NOT true of enantiomers? They have the same: |
| A. | Boiling point. |
| B. | Melting point. |
| C. | Specific rotation. |
| D. | Density. |
| Answer» C. Specific rotation. | |
| 19. |
Which statement is true of 1,3-dimethylcyclobutane?. |
| A. | Only one form of the compound is possible. |
| B. | Two diastereomeric forms are possible. |
| C. | Two sets of Enantiomers are possible. |
| D. | Two enantiomeric forms and one meso comp. |
| Answer» B. Two diastereomeric forms are possible. | |
| 20. |
CH3CHBrCH2CHClCH3 is the generalized representation of what number of stereo isomers? |
| A. | 3 |
| B. | 4 |
| C. | 5 |
| D. | 6 |
| Answer» B. 4 | |
| 21. |
For the generalized structure BrCH2CHClCH2CHClCH2Br there exists what number of stereoisomers? |
| A. | 2 |
| B. | 3 |
| C. | 4 |
| D. | 6 |
| Answer» C. 4 | |
| 22. |
How many discrete dimethylcyclopropanes are there? |
| A. | 2 |
| B. | 3 |
| C. | 4 |
| D. | 5 |
| Answer» C. 4 | |
| 23. |
What is the molecular formula for the alkenes of smallest molecular weight which possesses a stereogenic center? |
| A. | C4H10 |
| B. | C5H12 |
| C. | C6H14 |
| D. | C7H16 |
| Answer» D. C7H16 | |
| 24. |
How many chiral stereoisomers can be drawn for CH3CHFCHFCH (CH3)2? |
| A. | 1 |
| B. | 2 |
| C. | 3 |
| D. | 4 |
| Answer» D. 4 | |
| 25. |
How many different compounds are there which correspond to the general name "3-(1- methylbutyl) cyclobutanol”? |
| A. | 2 |
| B. | 4 |
| C. | 6 |
| D. | 8 |
| Answer» B. 4 | |
| 26. |
Which of the statements below correctly describes an achiral molecule? |
| A. | The molecule has a nonsuperimposable mirror image. |
| B. | The molecule exhibits optical activity when it interacts with plane-polarized light. |
| C. | The molecule has an enantiomer. |
| D. | The molecule might be a meso form. |
| Answer» D. The molecule might be a meso form. | |
| 27. |
Which of the following statements correctly pertains to a pair of enantiomers? |
| A. | They rotate the plane of polarized light by exactly the same amount and in opposite directions. |
| B. | They rotate the plane of polarized light by differing amounts and in opposite directions. |
| C. | They rotate the plane of polarized light by differing amounts and in the same direction. |
| D. | They have different melting points. |
| Answer» A. They rotate the plane of polarized light by exactly the same amount and in opposite directions. | |
| 28. |
A mixture of equal amounts of two enantiomers __________. |
| A. | is called a racemic mixture |
| B. | is optically inactive |
| C. | implies that the enantiomers are meso forms |
| D. | both A and B |
| Answer» D. both A and B | |
| 29. |
__________ is the use of an optically active reagent or catalyst to convert an optically inactive starting material into an optically active product. |
| A. | Asymmetric induction |
| B. | Racemization |
| C. | Optical reduction |
| D. | Meso effection |
| Answer» A. Asymmetric induction | |
| 30. |
Which of the following statements is (are) true for the compound (R)-2-butanol? |
| A. | This compound is chiral. |
| B. | This compound is optically active. |
| C. | This compound has an enantiomer. |
| D. | all of the above |
| Answer» D. all of the above | |
| 31. |
Which of the following statements is (are) true for the compound (3R, 4R)-3,4-dimethylhexane? |
| A. | This compound is chiral. |
| B. | The enantiomer of this compound is (3S, 4S)-3,4-dimethylhexane. |
| C. | This compound is a diastereomer of (3R, 4S)-3,4-dimethylhexane. |
| D. | all of the above |
| Answer» D. all of the above | |
| 32. |
Which of the following statements is (are) true for the compound cis-1,2-dichlorocyclopropane? |
| A. | This compound is chiral. |
| B. | The enantiomer of this compound is trans-1,2-dichlorocyclopropane. |
| C. | This compound contains no asymmetric carbons. |
| D. | none of the above |
| Answer» D. none of the above | |
| 33. |
How many asymmetric carbon atoms are present in the following compound?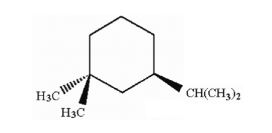
|
| A. | 0 |
| B. | 1 |
| C. | 2 |
| D. | 3 |
| Answer» B. 1 | |
| 34. |
How many chiral carbon atoms are present in the following compound?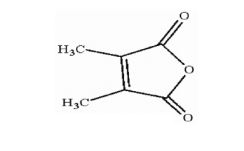
|
| A. | 0 |
| B. | 1 |
| C. | 2 |
| D. | 3 |
| Answer» A. 0 | |
| 35. |
Which of the following terms correctly describe(s) the structural relationship between cis-1,3- dimethylcyclopentane and trans-1,3-dimethylcyclopentane? |
| A. | enantiomers |
| B. | diastereomers |
| C. | geometric isomers |
| D. | both B and C |
| Answer» D. both B and C | |
| 36. |
How many diastereomers are there of the molecule shown below?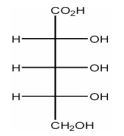
|
| A. | 0 |
| B. | 2 |
| C. | 4 |
| D. | 6 |
| Answer» D. 6 | |
| 37. |
How many enantiomers are there of the molecule shown below?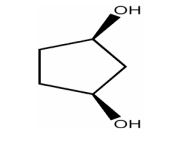
|
| A. | 0 |
| B. | 2 |
| C. | 4 |
| D. | 6 |
| Answer» A. 0 | |
| 38. |
What term describes the structural relationship between (E)- and (Z)-2-pentene? |
| A. | Constitutional isomers |
| B. | Enantiomers |
| C. | Geometric isomers |
| D. | Conformers |
| Answer» C. Geometric isomers | |
| 39. |
Compounds which have different arrangements of atoms in space while having same atoms bonded to each other are said to have |
| A. | position isomerism |
| B. | functional group isomerism |
| C. | chain isomerism |
| D. | stereoisomerism |
| Answer» D. stereoisomerism | |
| 40. |
Which of the following can make difference in optical isomers? |
| A. | heat |
| B. | temperature |
| C. | polarized light |
| D. | pressure |
| Answer» C. polarized light | |
| 41. |
Which of the following terms best describes the following pair of molecules?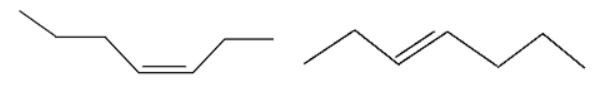
|
| A. | Isomers |
| B. | Constitutional isomers |
| C. | Configurational isomers |
| D. | Geometrical isomers |
| Answer» D. Geometrical isomers | |
| 42. |
What is the relationship between the two groups in the following molecules?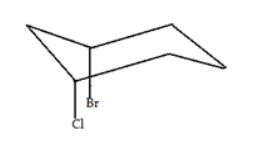
|
| A. | They are equatorial to one another |
| B. | They are axial to one another |
| C. | They are cis to one another |
| D. | They are trans to one another |
| Answer» A. They are equatorial to one another | |
| 43. |
What is the stereochemical relationship between the following two molecules?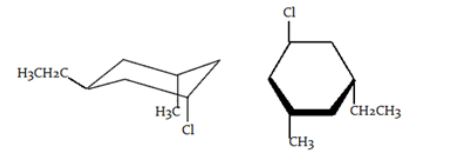
|
| A. | Geometrical isomers |
| B. | Enantiomers |
| C. | Diastereomers |
| D. | Identical |
| Answer» D. Identical | |
| 44. |
Which of the following is an alkane which can exhibit optical activity? |
| A. | Neopentane |
| B. | Isopentane |
| C. | 3–Methylpentane |
| D. | 3–Methylhexane |
| Answer» D. 3–Methylhexane | |
| 45. |
Hexane and 3-methylpentane are examples of: |
| A. | enantiomers. |
| B. | stereoisomers. |
| C. | diastereomers. |
| D. | constitutional isomers. |
| Answer» D. constitutional isomers. | |
| 46. |
Which of these is a comparatively insignificant factor affecting the magnitude of specific optical rotation. |
| A. | Concentration of substance of interest |
| B. | Temperature of measurement |
| C. | Length of the sample tube |
| D. | All of the above |
| Answer» D. All of the above | |
| 47. |
How many chiral stereoisomers can be drawn for CH3CHFCHFCH(CH3)2? |
| A. | 2 |
| B. | 4 |
| C. | 6 |
| D. | 8 |
| Answer» B. 4 | |
| 48. |
How many different compounds are there which correspond to the general name "3-(1- methylbutyl)cyclobutanol”? |
| A. | 2 |
| B. | 4 |
| C. | 6 |
| D. | 8 |
| Answer» B. 4 | |
| 49. |
Which of the following is NOT true of enantiomers? They have the same: |
| A. | boiling point. |
| B. | melting point. |
| C. | specific rotation. |
| D. | chemical reactivity toward achiral reagents. |
| Answer» D. chemical reactivity toward achiral reagents. | |
| 50. |
Which statement is true for 1,3-dimethylcyclobutane? |
| A. | Only one form of the compound is possible |
| B. | Two diastereomeric forms are possible. |
| C. | Two enantiomeric forms and one meso compound are possible. |
| D. | None of the above. |
| Answer» B. Two diastereomeric forms are possible. | |
Done Studing? Take A Test.
Great job completing your study session! Now it's time to put your knowledge to the test. Challenge yourself, see how much you've learned, and identify areas for improvement. Don’t worry, this is all part of the journey to mastery. Ready for the next step? Take a quiz to solidify what you've just studied.